
EU GMP Annex 1 – High quality meets high standards
Selecting the right cleanroom garments, eyewear, socks, and shoes is tricky, yet essential according to the EU GMP (Guidelines for Good Manufacturing Practice) Annex 1. With Lindström, you can get everything from the same provider.
Titled Manufacture of Sterile Medicinal Products, Annex 1 is a set of guidelines. They are designed to ensure the safety and quality of sterile medicinal products. An updated version was released in 2022. This version places an even greater emphasis on contamination control and the importance of personnel in preventing contamination.
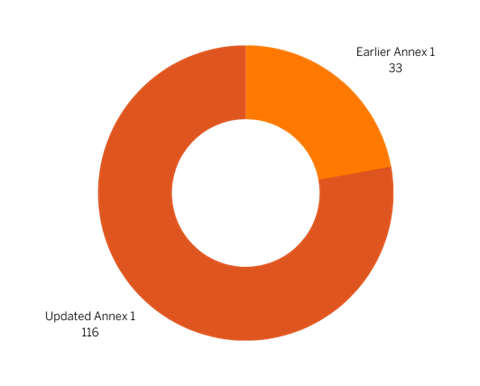
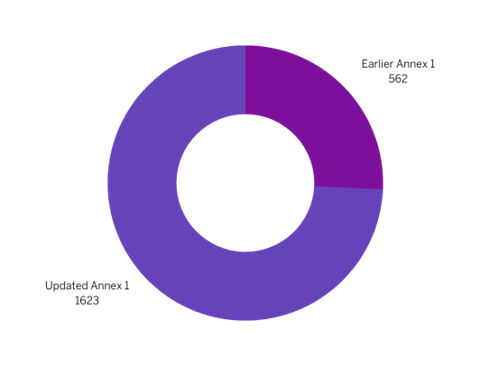
The updated Annex-1 guideline mentions Contamination 116 times. In comparison, it appeared only 33 times in the earlier version (See Figure 1). The Personnel section of the guideline has also increased from 562 to 1623 words (See Figure 2). This highlights the significance of personnel in maintaining sterile manufacturing environments.
The importance of training
The Good Manufacturing Practice (EU GMP) Guideline Annex 1, classified the area into four grades: A, B, C, and D. As an example, Grade A cleanrooms require a higher level of air quality than Grade B ones. Cleanroom garments and accessories must be appropriate for the process. Visually check the GMP grade of the working area for cleanliness and integrity before and after gowning. Ensure that garments and accessories minimize shedding due to operators’ movement, and replace them if identifying damage.
The guideline emphasizes the importance of personnel training, gowning qualification, and assessment in disciplines relevant to the correct manufacture of sterile products. All personnel performing cleaning, maintenance, monitoring, and accessing cleanrooms should receive regular training. This training should include the basic elements of microbiology and hygiene. It places specific focus on cleanroom practices, contamination control, aseptic techniques, and the protection of sterile products. The level of training should be based on the criticality of the function and area in which the personnel are working.
Train personnel accessing GMP grade A and B areas in aseptic gowning and aseptic behaviour. Assess and periodically reassess their compliance with aseptic gowning procedures at least annually. High standards of personal hygiene and cleanliness are essential to prevent excessive shedding. It also decreases the risk of introduction of microbial contamination. Personnel involved in the manufacturing of sterile products should have instructions to report any specific health conditions or ailments that may cause the shedding of abnormal numbers or types of contaminants. For instance, respiratory droplets or flakes of skin that therefore preclude cleanroom access.
To summarise EU GMP Annex 1
The EU GMP Guideline Annex 1 plays a vital role in ensuring the safety and quality of sterile medicinal products. The updated version places a greater emphasis on contamination control and the role of personnel in preventing contamination. The guideline highlights the importance of selecting the right cleanroom garments, personnel training, and maintaining high standards of personal hygiene and cleanliness. Manufacturers must follow these guidelines to ensure the safety and quality of their products and protect the health of patients.
To support the pharmaceutical industry engaged in manufacturing sterile products, Lindström provides high-quality cleanroom services with compliant garments and accessories. They meet the required cleanliness standards, ensuring a hygienic workplace environment.



